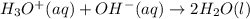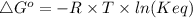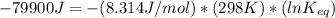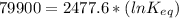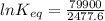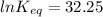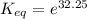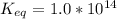Chemistry, 14.07.2019 10:30 samantha636
The standard free energy change for the formation of two moles of h2o(l) in a strong acid–strong base neutralization reaction at 25°c is -79.9kj. calculate the equilibrium constant for the reaction. see equation 11.1. h3o+(aq) + oh-(aq) = 2 h2o (l)

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 06:40
Three alkali metals in group 1 are a. calcium, strontium, barium b. boron, aluminum, gallium c. sodium, potassium, rubidium d. fluorine, iodine, chlorine
Answers: 1

Chemistry, 22.06.2019 11:40
Which type of precipitation would most likely form when the surface air temperature is slightly below freezing and the air temperature increases as you move upward away from the ground?
Answers: 2

Chemistry, 22.06.2019 12:30
The melting point of sulfur is 115 °c and its boiling point is 445 °c. what state would sulfur be in at 200 °c?
Answers: 1

Chemistry, 22.06.2019 19:20
15. which of the following is not human-caused groundwater pollution? a. water in an aquifer dissolves elements such as arsenic and mercury from surrounding rock. b. water in an aquifer is contaminated by leachate that seeps into the ground from a landfill. c. water in an aquifer becomes polluted with chemicals used in hydraulic fracturing, or fracking. d. water in an aquifer absorbs harmful bacteria from the drainage field of a septic tank.
Answers: 1
You know the right answer?
The standard free energy change for the formation of two moles of h2o(l) in a strong acid–strong bas...
Questions


History, 04.11.2020 20:20


Geography, 04.11.2020 20:20

Mathematics, 04.11.2020 20:20

Spanish, 04.11.2020 20:20

History, 04.11.2020 20:20

Chemistry, 04.11.2020 20:20



English, 04.11.2020 20:20

Mathematics, 04.11.2020 20:20

Mathematics, 04.11.2020 20:20

Mathematics, 04.11.2020 20:20


Mathematics, 04.11.2020 20:20




Computers and Technology, 04.11.2020 20:20