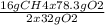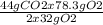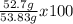Consider the following reaction. ch4 (g) + o2 (g) co2 (g) + h2o (l) a chemist allows 23.2g of ch4 and 78.3g o2 to react. when the reaction is finished, the chemist collects 52.7g co2. determine the limiting reagent, theoretical yield, and percent yield for the reaction

Answers: 1


Another question on Chemistry

Chemistry, 20.06.2019 18:04
For the following reaction, 5.65 grams of oxygen gas are mixed with excess hydrochloric acid . assume that the percent yield of water is 86.4 %. hydrochloric acid(aq) + oxygen(g) water(l) + chlorine(g) what is the ideal yield of water ? grams what is the actual yield of water ? grams
Answers: 1



Chemistry, 22.06.2019 13:10
Select the correct answer a modure consists of glucose and water. what is the percent composition of glucose in the mixture if it contains 1.3 moles of glucose (cho total mass of the mature is 276 grams? ) and the a 1775
Answers: 1
You know the right answer?
Consider the following reaction. ch4 (g) + o2 (g) co2 (g) + h2o (l) a chemist allows 23.2g of ch4 an...
Questions


Mathematics, 05.02.2021 21:10




Physics, 05.02.2021 21:10

Mathematics, 05.02.2021 21:10


History, 05.02.2021 21:10



History, 05.02.2021 21:10


Mathematics, 05.02.2021 21:10


Biology, 05.02.2021 21:10

Mathematics, 05.02.2021 21:10


Biology, 05.02.2021 21:10

History, 05.02.2021 21:10