
Chemistry, 14.07.2019 13:00 devenybates
At which temperature would a reaction with h = -92 kj/mol, s = -0.199 kj/(molk) be spontaneous?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 23:00
Matches the chemical name of each oxide of phosphorus to its chemical formula
Answers: 2


Chemistry, 22.06.2019 08:30
Agroup of students is studying convection current. they fill two identical balloons with the same amount of helium. one balloon is placed in a freezer and the other is in an area with warm air. after 10 minutes, the balloon are released from a height of 1 meter. which of the following to the students most likely observe? a) the warm balloon expands and rises. the cold balloon shrinks and sinks b) the balloon both rise. the cold balloon is larger than the warm balloon c) the cold balloon expands and rises. the warm balloon shrinks and sinks d) the balloon rise at the same rate. both balloons are the same size
Answers: 1

Chemistry, 22.06.2019 09:00
Given the following reaction: c3h8+5o2=3co2+4h20 how many grams of co2 will be produced 7 g of c3h8 and 98 g of o2
Answers: 1
You know the right answer?
At which temperature would a reaction with h = -92 kj/mol, s = -0.199 kj/(molk) be spontaneous?...
Questions


Mathematics, 02.10.2020 17:01

Chemistry, 02.10.2020 17:01

Biology, 02.10.2020 17:01

Mathematics, 02.10.2020 17:01

Computers and Technology, 02.10.2020 17:01

Mathematics, 02.10.2020 17:01


English, 02.10.2020 17:01



History, 02.10.2020 17:01


Social Studies, 02.10.2020 17:01



History, 02.10.2020 17:01


English, 02.10.2020 17:01

Mathematics, 02.10.2020 17:01

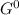 = Δ
= Δ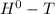 Δ
Δ
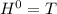 Δ
Δ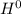 , giving a negative value for Δ
, giving a negative value for Δ 

