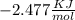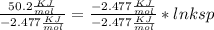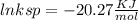Chemistry, 14.07.2019 13:30 Yorlin4441
Use the thermodynamic data at 298 k below to determine the ksp for barium carbonate, baco3 at this temperature. substance: ba2+(aq) co32–(aq) baco3(s) δh°f (kj/mol): –538.36 –676.26 –1219 δg°f (kj/mol): –560.7 –528.1 –1139 s°(j/k·mol): 13 –53.1 112

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 08:00
Which of the following observations indicates that there is a small, dense, positively charged part in the center of an atom? some uncharged particles are scattered by a gold foil. all uncharged particles are attracted towards a gold foil. all positively charged particles pass straight through a gold foil. some positively charged particles bounce back from a gold foil.
Answers: 2

Chemistry, 22.06.2019 08:30
What is the independent variable in this investigation? mass volume sample number substance density
Answers: 3

Chemistry, 22.06.2019 18:00
Alidded glass container is filled with a colored gas. after a period of time, it is observed that the gas is uniformly spread throughout the box and that the movement has slowed considerably. next, a warm iron plate is carefully placed under the box. why is there resumed movement of the gas in the container?
Answers: 2

Chemistry, 22.06.2019 20:20
The characteristics of two different types of reactions are shown below: reaction a: electrons are gained by the atoms of an element. reaction b: protons are lost by the atom of an element. which statement is true about the atoms of the elements that participate in the two reactions? their identity changes in both reaction a and reaction b. their identity changes in reaction a but not in reaction b. their identity changes in reaction b but not in reaction a. their identity remains the same in both reaction a and reaction b.
Answers: 1
You know the right answer?
Use the thermodynamic data at 298 k below to determine the ksp for barium carbonate, baco3 at this t...
Questions

Physics, 20.09.2019 23:30






World Languages, 20.09.2019 23:30

Geography, 20.09.2019 23:30

Computers and Technology, 20.09.2019 23:30

Chemistry, 20.09.2019 23:30



Biology, 20.09.2019 23:30




English, 20.09.2019 23:30



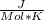
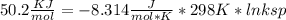
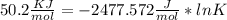
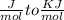
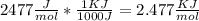 ))
))