Chemistry, 14.07.2019 15:00 Nickanderson21
According to the first law of thermodynamics, the amount of work done by a heat engine equals the amount of a. work done on the engine. b. waste heat it produces. c. thermal energy added to the engine minus the waste heat. d. thermal energy added to the engine plus the waste heat.

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 10:00
Water's surface tension and heat storage capacity are accounted for by its a) orbitals b) weight c) hydrogen bonds d) mass e) size
Answers: 2

Chemistry, 22.06.2019 10:10
For the reaction, 4 a(g) + 3 b(g) => 2 c(g), the following data were obtained at constant temperature. experiment initial[a],mol/l initial [b],mol/l initial rate,m/min 1 0.200 0.150 5.00 2 0.400 0.150 10.0 3 0.200 0.300 10.0 4 0.400 0.300 20.0 which of the following is the correct rate law for the reaction? 1. rate = k[a]2[b]2 2. rate = k[a][b] 3. rate = k[a]2[b] 4. rate = k[a][b]2
Answers: 3

Chemistry, 22.06.2019 13:30
What produces wave a)sound b) heats c)transfer of energy d)vibrations
Answers: 2
You know the right answer?
According to the first law of thermodynamics, the amount of work done by a heat engine equals the am...
Questions

Biology, 09.12.2021 01:00


Social Studies, 09.12.2021 01:00

Biology, 09.12.2021 01:00



Geography, 09.12.2021 01:00


Mathematics, 09.12.2021 01:00

Mathematics, 09.12.2021 01:00


Mathematics, 09.12.2021 01:00






History, 09.12.2021 01:00

English, 09.12.2021 01:00

History, 09.12.2021 01:00

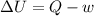
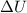 = Internal energy
= Internal energy