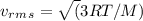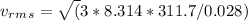Chemistry, 14.07.2019 16:30 emmagbales
Find the rms speed of the molecules of a sample of n2 (diatomic nitrogen) gas at a temperature of 38.7°c.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:30
Use the periodic table to determine the electron configuration of dysprosium (dy) and americium (am) in noble-gas notation.
Answers: 1


Chemistry, 22.06.2019 16:40
Let the ed50 of a recreational drug be defined as the amount required for 50% of a test group to feel high or get a buzz. if the ed50 value of ethanol is 470 mg/kg body mass, what dose would a 70 kg party goer need to quickly consume in order to have a 50% chance of getting a buzz? 235 mg 470 mg 32,900 mg 35,000,000 mg
Answers: 3

Chemistry, 22.06.2019 19:00
A4.86 g piece of metal was placed in a graduated cylinder containing 15.5 ml of water. the water level rose to 17.3 ml. what is the density of the metal. i need the steps of how to solve it to so i can use a formula to work out other problems.
Answers: 1
You know the right answer?
Find the rms speed of the molecules of a sample of n2 (diatomic nitrogen) gas at a temperature of 38...
Questions

History, 29.08.2019 08:50

Social Studies, 29.08.2019 08:50

Social Studies, 29.08.2019 08:50

History, 29.08.2019 08:50

History, 29.08.2019 08:50

History, 29.08.2019 08:50


English, 29.08.2019 08:50

History, 29.08.2019 08:50

Mathematics, 29.08.2019 08:50

History, 29.08.2019 08:50

Mathematics, 29.08.2019 08:50



English, 29.08.2019 08:50


Mathematics, 29.08.2019 08:50