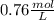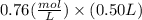Chemistry, 14.07.2019 16:30 thomasmurphy200
The benzoate ion, c6h5coo− is a weak base with kb=1.6×10−10. how many moles of sodium benzoate are present in 0.50 l of a solution of nac6h5coo if the ph is 9.04?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Agas at 155 kpa and standard temperature has an initial volume of 1.00 l. the pressure of the gas rises to 500 kpa as the temperature also rises to 135°c. what is the new volume? 2.16 l 0.463 l 0.207 l 4.82 l
Answers: 3

Chemistry, 22.06.2019 17:00
The atoms of a solid aluminum can are close together, vibrating in a rigid structure. if the can is warmed up on a hot plate, what happens to the atoms?
Answers: 1

Chemistry, 23.06.2019 01:30
Which of the following statements is true about energy quantization at the atomic level? electrons in the outermost orbits are the most stable. electrons in all the orbits around the nucleus have the same amount of energy. electrons in the orbit closest to the nucleus have the least amount of energy. electrons absorb or release the same amount of energy independent of the energy levels.
Answers: 1

Chemistry, 23.06.2019 04:00
What two categories of toxins were present in the air at dish,texas as a result of the gas pipelines that pass through the area
Answers: 1
You know the right answer?
The benzoate ion, c6h5coo− is a weak base with kb=1.6×10−10. how many moles of sodium benzoate are p...
Questions


English, 14.12.2021 20:40




Mathematics, 14.12.2021 20:40

Mathematics, 14.12.2021 20:40

Biology, 14.12.2021 20:40

Mathematics, 14.12.2021 20:40



History, 14.12.2021 20:40

Mathematics, 14.12.2021 20:40


History, 14.12.2021 20:40


English, 14.12.2021 20:40


Spanish, 14.12.2021 20:40

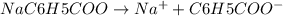
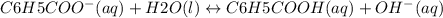
![[OH^{-}] = 10^{^{-pOH}}](/tpl/images/0089/2713/549cf.png)
![[OH^{-}] = 10^{^{-4.96}}](/tpl/images/0089/2713/ff5c3.png)
![[OH^{-}] = 1.1\times 10^{-5}](/tpl/images/0089/2713/97cf5.png)
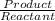
![kb =\frac{[C6H5COOH][OH^{-}]}{[C6H5COO^{-}]}](/tpl/images/0089/2713/6f928.png)
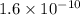
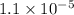 and value of C6H5COOH is equal to OH-
and value of C6H5COOH is equal to OH-![1.6\times 10^{-10} = \frac{[1.1\times 10^{-5}][1.1\times 10^{-5}]}{[C6H5COO^{-}]}](/tpl/images/0089/2713/af234.png)
![[C6H5COO^{-}] = \frac{[1.1\times 10^{-5}][1.1\times 10^{-5}]}{1.6\times 10^{-10}}](/tpl/images/0089/2713/c4847.png)
![[C6H5COO^{-}] = 0.76 M](/tpl/images/0089/2713/e77f3.png) or
or