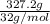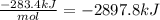Chemistry, 14.07.2019 18:00 AleciaCassidy
Solid sodium peroxide (na2o2) reacts with liquid water yielding aqueous sodium hydroxide and oxygen gas. how much heat is released if 327.2 g of oxygen gas is produced from the reaction of sodium peroxide and water under standard-state conditions?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 09:00
This chart lists four kinds of polymers and their sources. what can be known about all four polymers, despite their differences? they come from living things. they share ionic carbon bonds. they are at least 100 monomers long. they are made of repeating subunits.
Answers: 1

Chemistry, 22.06.2019 12:00
An atom's configuration based on its number of electrons ends at 3p4. another atom has seven more electrons. starting at 3p, what is the remaining configuration? 3p63d34s2 3p43d54s2 3p64s23d3 3p44s23d
Answers: 3

Chemistry, 22.06.2019 18:00
Heat is the total potential energy of a substance that can be transferred. true false
Answers: 1

Chemistry, 22.06.2019 23:00
Which organism develops breathing organism develops breathing organs from pharyngeal arches? shark, spider, sea star, sea horse
Answers: 2
You know the right answer?
Solid sodium peroxide (na2o2) reacts with liquid water yielding aqueous sodium hydroxide and oxygen...
Questions



Spanish, 06.06.2021 07:00


Mathematics, 06.06.2021 07:00

Chemistry, 06.06.2021 07:00


Mathematics, 06.06.2021 07:00


Mathematics, 06.06.2021 07:00

Mathematics, 06.06.2021 07:00

Mathematics, 06.06.2021 07:00


Social Studies, 06.06.2021 07:10

Computers and Technology, 06.06.2021 07:10


Chemistry, 06.06.2021 07:10

Advanced Placement (AP), 06.06.2021 07:10

Computers and Technology, 06.06.2021 07:10

Mathematics, 06.06.2021 07:10