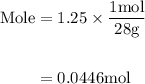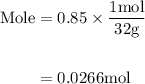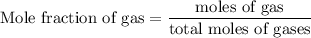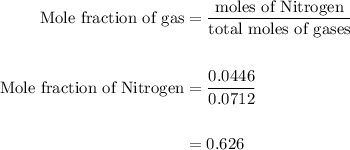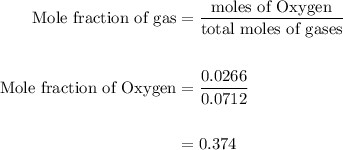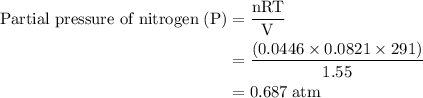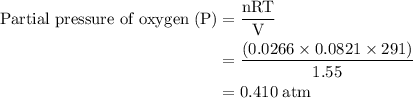Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 07:30
The table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 10 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of the two atoms? both are unreactive. both are highly reactive. a is unreactive and d is reactive. a is reactive and d is unreactive.
Answers: 3

Chemistry, 22.06.2019 09:20
How have the greenhouse gasses increased from the year 2000 to 2018
Answers: 2

Chemistry, 22.06.2019 12:30
If 22.5 liters of oxygen reacted with excess of hydrogen, how many liters of water vapor could be produced?
Answers: 3

Chemistry, 22.06.2019 19:30
What is the common name for the compound shown here? enter the common name of the compound shown?
Answers: 2
You know the right answer?
Agas mixture contains 1.25 g n2 and 0.85 g o2 in a 1.55 l c ontainer at 18 °c. calculate the mole fr...
Questions

History, 20.05.2021 05:30






Mathematics, 20.05.2021 05:30

Mathematics, 20.05.2021 05:30


Mathematics, 20.05.2021 05:30







Mathematics, 20.05.2021 05:30