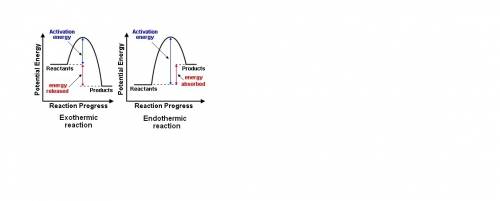
How does the potential-energy diagram for a reaction indicate whether the reaction is endothermic or exothermic? an endothermic reaction has reactants that are lower in energy than products because energy is absorbed to form the products. an endothermic reaction has reactants that are higher in energy than products because energy is released to form the products. an exothermic reaction has reactants that are lower in energy than products because energy is released to form the products. an exothermic reaction has reactants that are higher in energy than products because energy is absorbed to form the products.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 18:50
Which term refers to a property that depends only on the amount of a substance? ©@
Answers: 2

Chemistry, 21.06.2019 22:30
Often on a topographic map, every fifth contour line is darkened. what is this line called? a. key b.slope c.benchmark d. index contour
Answers: 1

Chemistry, 22.06.2019 10:00
Diffraction is when light is bent around obstructions. which of the these observation about clouds would indicate diffraction? a) after rain storms, you can sometimes see rainbows. b) clouds are white or gray and cannot be seen through. c) on a cloudy day, the temperature tends to be cooler than a sunny day. d) the edges of dark clouds appear lighter. this
Answers: 3

Chemistry, 22.06.2019 22:30
Amedication is given at a dosage of 3.000 mg of medication per kg of body weight. if 0.1500 g of medication is given, then what was the patient's weight in pounds (lbs)? there are 453.59g in 1 lb.
Answers: 2
You know the right answer?
How does the potential-energy diagram for a reaction indicate whether the reaction is endothermic or...
Questions

Biology, 08.01.2021 20:00


Social Studies, 08.01.2021 20:00

Mathematics, 08.01.2021 20:00


History, 08.01.2021 20:00



History, 08.01.2021 20:00

Mathematics, 08.01.2021 20:00

English, 08.01.2021 20:00

Mathematics, 08.01.2021 20:00

English, 08.01.2021 20:00

Mathematics, 08.01.2021 20:00


Chemistry, 08.01.2021 20:00

Mathematics, 08.01.2021 20:00

Advanced Placement (AP), 08.01.2021 20:00

Mathematics, 08.01.2021 20:00

Geography, 08.01.2021 20:00