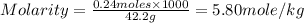Chemistry, 14.07.2019 19:30 MarishaTucker
Calculate the molality of a solution containing 14.3 g of nacl in 42.2 g of water. a. 2.45 ï´ 10â4 m b. 5.80 ï´ 10â4 m c. 2.45 ï´ 10â1 m d. 103 m e. 5.80 m

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 05:10
How many miles of water are produced if 5.43 mol pbo2 are consumed
Answers: 1

Chemistry, 22.06.2019 06:30
What is the correct term for living the most sustainable life you can within your current circumstances?
Answers: 1

Chemistry, 22.06.2019 20:00
Many free radicals combine to form molecules that do not contain any unpaired electrons. the driving force for the radical–radical combination reaction is the formation of a new electron‑pair bond. consider the chemical equation. n(g)+no(g)⟶nno(g) n(g)+no(g)⟶nno(g) write lewis formulas for the reactant and product species in the chemical equation. include nonbonding electrons. n(g)n(g) select draw rings more erase select draw rings more erase select draw rings more erase n no(g)
Answers: 1

Chemistry, 22.06.2019 22:30
Which process describes vaporization that takes place below the surface of a liquid? condensation melting boiling evaporation
Answers: 1
You know the right answer?
Calculate the molality of a solution containing 14.3 g of nacl in 42.2 g of water. a. 2.45 ï´ 10â4 m...
Questions

Mathematics, 30.09.2019 17:50

Health, 30.09.2019 17:50

Mathematics, 30.09.2019 17:50

Physics, 30.09.2019 17:50



Mathematics, 30.09.2019 17:50

Social Studies, 30.09.2019 17:50


Mathematics, 30.09.2019 17:50

Computers and Technology, 30.09.2019 17:50


English, 30.09.2019 17:50

English, 30.09.2019 17:50


Geography, 30.09.2019 17:50

English, 30.09.2019 17:50

History, 30.09.2019 17:50


Chemistry, 30.09.2019 17:50

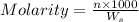
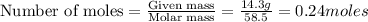
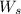 = weight of solvent in g= 42.2 g
= weight of solvent in g= 42.2 g