

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 07:30
In a reaction (at equilibrium) that makes more moles of gas than it consumes, what is the effect of increasing the pressure?
Answers: 1


Chemistry, 23.06.2019 05:40
Why is it incorrect to balance a chemical equation by changing the subscripts? explain.
Answers: 2

Chemistry, 23.06.2019 06:40
8. how much enthalpy/heat is transferred when 0.5113gof ammonia (nh3) reacts with excess oxygen according| to the following equation: 4nh3 +502 - 4n0+ 6h20ah = -905.4j
Answers: 1
You know the right answer?
Assuming the volume of the insoluble substance to be 92.5 ml and the mass to be 693.8 g, what is the...
Questions








Mathematics, 22.02.2020 01:00


Biology, 22.02.2020 01:00





Computers and Technology, 22.02.2020 01:00





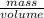
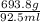 = 7.50 g/ml
= 7.50 g/ml

