

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 15:30
Using the first volume and temperature reading on the table as v1 and t1, solve for the unknown values in the table below. remember to use the rules of significant figures when entering your numeric response.
Answers: 2

Chemistry, 22.06.2019 20:00
Acm ruler with main graduations from 1 to 10 from left to right there are 10 secondary graduations between each of the main graduations there is a line that begins. at the left end of the ruler 10 secondary graduations to the left of the “1 main graduation the right end of the line ends on the eighth secondary graduation to the right of 3 how long is the line
Answers: 1

Chemistry, 23.06.2019 01:10
A5.00 g of a in . g of at aa 5.00 g of b in . g of .?at .
Answers: 1
You know the right answer?
How many moles of ag can be produced if 350. g of cu are reacted with excess agno3 according to the...
Questions

Mathematics, 26.02.2021 18:40

Mathematics, 26.02.2021 18:40


Mathematics, 26.02.2021 18:40


Mathematics, 26.02.2021 18:40



Mathematics, 26.02.2021 18:40

Mathematics, 26.02.2021 18:40



Mathematics, 26.02.2021 18:40

Physics, 26.02.2021 18:40

Mathematics, 26.02.2021 18:40





Mathematics, 26.02.2021 18:40

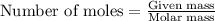


 of silver.
of silver.

