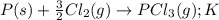Chemistry, 15.07.2019 13:00 pablogonzaleztellez
Consider the reaction: p(s) + 3/2 cl2( g. pcl3( g. write the equilibrium constant for this reaction in terms of the equilibrium constants, ka and kb, for reactions a and b below: a.)p(s) + 5/2 cl2( g. pcl5( g. ka b.)pcl3( g. + cl2( g. pcl5( g. kb

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 06:00
If you burn 10 kilograms of wood in a fire (combustion) what is the weight of the products after the fire has finished burning the wood?
Answers: 3

Chemistry, 22.06.2019 08:30
In the millikan oil drop experiment they determined that every drop had a charge which was a while number multiple of -1.60x10^-19. if a drop has a total charge of -9.60x10^-19 then how many excess electrons are contained within the drop?
Answers: 2

Chemistry, 22.06.2019 09:40
Which diagram shows the correct way to represent an ionic compound of magnesium oxide?
Answers: 3

Chemistry, 22.06.2019 09:50
Although respiratory organs vary across different organisms, they all contain respiratory surfaces that have a large surface area and are extremely thin. explain why having an extremely thin respiratory surface with a large surface area is advantageous for the process of gas exchange
Answers: 1
You know the right answer?
Consider the reaction: p(s) + 3/2 cl2( g. pcl3( g. write the equilibrium constant for this reaction...
Questions




Mathematics, 21.04.2021 21:00

Mathematics, 21.04.2021 21:00

English, 21.04.2021 21:00


Mathematics, 21.04.2021 21:00

English, 21.04.2021 21:00







Computers and Technology, 21.04.2021 21:00


English, 21.04.2021 21:00


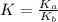
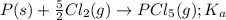
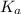 for the above equation is:
for the above equation is:![K_a=\frac{[PCl_5]}{[P][Cl_2]^{5/2}}](/tpl/images/0092/5571/bd7b7.png) ......(1)
......(1)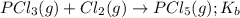
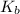 for the above equation is:
for the above equation is:![K_b=\frac{[PCl_5]}{[PCl_3][Cl_2]}](/tpl/images/0092/5571/bd7b0.png) ......(2)
......(2)![\frac{K_a}{K_b}=\left(\frac{\frac{[PCl_5]}{[P][Cl_2]^{5/2}}}{\frac{[PCl_5]}{[PCl_3][Cl_2]}}\right)\\\\\\\frac{K_a}{K_b}=\frac{[PCl_3]}{[P][Cl_2]^{3/2}}](/tpl/images/0092/5571/fcd9a.png)