
Chemistry, 15.07.2019 15:30 kikidsgn123
It takes 238./kjmol to break a carbon-iodine single bond. calculate the maximum wavelength of light for which a carbon-iodine single bond could be broken by absorbing a single photon. round your answer to 3 significant digits.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:20
Calcium hydride (cah2) reacts with water to form hydrogen gas: cah2(s) + 2h2o(l) → ca(oh)2(aq) + 2h2(g) how many grams of cah2 are needed to generate 45.0 l of h2 gas at a pressure of 0.995 atm and a temperature of 32 °c?
Answers: 2

Chemistry, 22.06.2019 16:10
Amixture initially contains a, b, and c in the following concentrations: [a] = 0.300 m , [b] = 1.05 m , and [c] = 0.550 m . the following reaction occurs and equilibrium is established: a+2b⇌c at equilibrium, [a] = 0.140 m and [c] = 0.710 m . calculate the value of the equilibrium constant, kc.
Answers: 1

Chemistry, 22.06.2019 20:00
For the reaction c6h14(g) & longrightarrow; c6h6(g) + 4h2(g), δp(h2)/δt was found to be 2.5 x 10-2 atm/s, where δp(h2) is the change in pressure of hydrogen. determine δp(c6h14)/δt for this reaction at the same time.
Answers: 2

You know the right answer?
It takes 238./kjmol to break a carbon-iodine single bond. calculate the maximum wavelength of light...
Questions


Mathematics, 15.04.2021 06:30

Mathematics, 15.04.2021 06:30





History, 15.04.2021 06:30

Mathematics, 15.04.2021 06:30


Social Studies, 15.04.2021 06:30

Mathematics, 15.04.2021 06:30

Mathematics, 15.04.2021 06:30





Mathematics, 15.04.2021 06:30


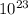 ) = 3.953
) = 3.953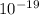 J
J 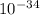 Js X 2.9979
Js X 2.9979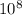 m/s) / (3.953
m/s) / (3.953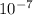 m
m
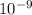 m
m


