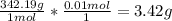Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 07:30
All cells are made of four types of acids, lipids, proteins, and carbohydrates.
Answers: 1

Chemistry, 22.06.2019 10:20
In a reaction equation, where are the products located? a.) above the arrow b.) to the right of the arrow c.) to the left of the arrow d.) below the arrow
Answers: 2


Chemistry, 22.06.2019 16:00
How could a student test the effect of removing heat from a gas that is stored in a sealed container? what must occur in order for matter to change states?
Answers: 2
You know the right answer?
First: calculate the number of moles of kool-aid powder needed to make 100ml of a 0.1m solution. 0....
Questions

Health, 01.07.2019 11:30




Chemistry, 01.07.2019 11:30



Mathematics, 01.07.2019 11:30


English, 01.07.2019 11:30


Mathematics, 01.07.2019 11:30

History, 01.07.2019 11:30

Mathematics, 01.07.2019 11:30

English, 01.07.2019 11:30

Mathematics, 01.07.2019 11:30

English, 01.07.2019 11:30


Mathematics, 01.07.2019 11:30

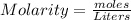
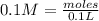
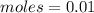
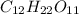 .
.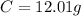
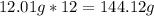
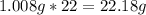
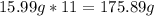
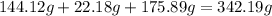 -> So we know that the molar weight of the Kool-Aid powder is 342.19g.
-> So we know that the molar weight of the Kool-Aid powder is 342.19g.