
Chemistry, 15.07.2019 17:00 imoreno8412
Calculate the enthalpy change for the thermite reaction: 2al(s)+fe2o3(s)→2fe(s)+al2o3(s), δh∘rxn=−850 kj when 12.0 mol of al undergoes the reaction with a stoichiometrically equivalent amount of fe2o3.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 01:00
Which of the following is not a true statement about dwarf planets? a the kuiper belt contains comets, asteroids, and dwarf planets. b ceres is a dwarf planet located in the kuiper belt. c the largest known dwarf planet in the solar system is named eris.
Answers: 2

Chemistry, 22.06.2019 05:30
Describe the interaction that occurs between two objects with the same electrical charge.
Answers: 1

Chemistry, 22.06.2019 12:00
Solutions of sodium carbonate and silver nitrate react to form solid silver carbonate and a solution of sodium nitrate. a solution containing 3.50 g of sodium carbonate is mixed with one containing 5.00 g of silver nitrate. how many grams of sodium carbonate, silver nitrate, silver carbonate, and sodium nitrate are present after the reaction is complete?
Answers: 2

Chemistry, 22.06.2019 12:30
Avariable that is not being directly tested during an experiment should be changed varied experimented controlled
Answers: 1
You know the right answer?
Calculate the enthalpy change for the thermite reaction: 2al(s)+fe2o3(s)→2fe(s)+al2o3(s), δh∘rxn=−8...
Questions

Biology, 12.10.2020 21:01


Mathematics, 12.10.2020 21:01




Mathematics, 12.10.2020 21:01


English, 12.10.2020 21:01

Chemistry, 12.10.2020 21:01

Biology, 12.10.2020 21:01

Arts, 12.10.2020 21:01

Mathematics, 12.10.2020 21:01

History, 12.10.2020 21:01


Mathematics, 12.10.2020 21:01

Mathematics, 12.10.2020 21:01


History, 12.10.2020 21:01

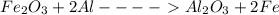
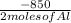 = -5100 KJ
= -5100 KJ

