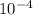Chemistry, 15.07.2019 19:00 lerasteidl
The equilibrium constant, keq, is 4.4 × 10-4 at 500 k. 2nocl (g) < --> 2no(g) + cl2 (g) determine the direction of reaction if 1.00 m nocl is combined with 0.500 m no and 0.500 m cl2 in a sealed flask. a.)no reaction occurs. b.)reaction proceeds to the right toward the products. c.)reaction proceeds to the left toward the reactants. d.)reaction proceeds to the left toward the products.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 02:10
When 225mg of anthracene, c14h10(s), was burned in a bomb calorimeter the temperature rose by 1.75k. calculate the calorimeter constant. by how much will the temperature rise when 125mg of phenol, c6h5oh(s), is burned in the calorimeter under the same conditions? (δch< (c14h10,s)=–7061 kj mol−1.)
Answers: 3

Chemistry, 22.06.2019 04:40
Silver tarnishes as silver metal reacts with hydrogen sulfide, h2s, in the air. in this reaction, dark silver sulfide, au2s, covers the surface of silver. when silver is polished, this coating of silver sulfide can be removed from the surface. this makes the silver shiny again. enter the coefficients that balance the tarnishing reaction equation. (type 1 for no coefficient.)
Answers: 2

Chemistry, 22.06.2019 13:10
Which electron configuration represents the electrons in an atom of sodium in the ground state at stp
Answers: 1

Chemistry, 22.06.2019 19:30
Acetylene gas c2h2 undergoes combustion to produce carbon dioxide and water vapor how many grams of water are produced by the same amount of c2h2?
Answers: 2
You know the right answer?
The equilibrium constant, keq, is 4.4 × 10-4 at 500 k. 2nocl (g) < --> 2no(g) + cl2 (g) deter...
Questions

Mathematics, 30.10.2019 23:31




Geography, 30.10.2019 23:31


Social Studies, 30.10.2019 23:31


Social Studies, 30.10.2019 23:31


Mathematics, 30.10.2019 23:31


Geography, 30.10.2019 23:31

Biology, 30.10.2019 23:31

Mathematics, 30.10.2019 23:31


Computers and Technology, 30.10.2019 23:31


Chemistry, 30.10.2019 23:31

Biology, 30.10.2019 23:31

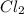
![Q = \frac{[ NO]^{2}. [Cl_{2} ]}{[ NOCl ]^{2}}](/tpl/images/1058/5527/aa67c.png)