Chemistry, 15.07.2019 19:30 CoolRahim9090
Calculate the standard molar enthalpy for the complete combustion of liquid ethanol (c2h5oh) using the standard enthalpies of formation of the reactants and products.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 10:30
Great amounts of electromagnetic energy from our sun and other bodies in space travel through space. which is a logical conclusion about these electromagnetic waves? their energy must be very their frequency must be very low these waves can travel without a medium they only travel through a vacuum of space
Answers: 2

Chemistry, 22.06.2019 16:50
Assuming complete dissociation of the solute, how many grams of kno3 must be added to 275 ml of water to produce a solution that freezes at -14.5 c? the freezing point for pure water is 0.0 c and k_f is equal to 1.86 c/m
Answers: 3

Chemistry, 22.06.2019 19:50
If a gas has an initial pressure of 101kpa and a volume of 10l, then it expands to a volume of 20l, what is the new pressure?
Answers: 2

Chemistry, 23.06.2019 15:00
In two or more complete sentences describe all of the van der waals forces that exist between molecules of sulfur dioxide, so2.
Answers: 1
You know the right answer?
Calculate the standard molar enthalpy for the complete combustion of liquid ethanol (c2h5oh) using t...
Questions

Mathematics, 11.09.2021 02:10

Mathematics, 11.09.2021 02:10

English, 11.09.2021 02:10

Mathematics, 11.09.2021 02:10

English, 11.09.2021 02:10


History, 11.09.2021 02:10

Mathematics, 11.09.2021 02:10

Biology, 11.09.2021 02:10

Arts, 11.09.2021 02:10

Mathematics, 11.09.2021 02:10




Mathematics, 11.09.2021 02:10




Social Studies, 11.09.2021 02:10

Health, 11.09.2021 02:10

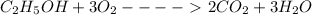
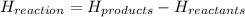
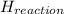 =
=