
Chemistry, 15.07.2019 20:30 Badbpyz7550
Calculate the heat released when 5.00 l of cl2(g) with a density of 1.88 g/l reacts with an excess of sodium metal at 25°c and 1 atm to form sodium chloride.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 23:00
Select the correct answer. given: 2libr + ba → babr2 + 2li in this chemical reaction, 325 grams of barium (ba) react completely. how many moles of lithium (li) are produced? a. 1.18 mol b. 2.37 mol c. 4.73 mol d. 16.4 mol e. 32.9 mol
Answers: 2

Chemistry, 22.06.2019 03:30
If a solution is considered basic, then a) the hydroxide ion and hydronium ion concentrations are equal. b) the hydroxide ion concentration is less than the hydronium ion concentration. c) the hydronium ion concentration is greater than the hydroxide ion concentration. d) the hydroxide ion concentration is greater than the hydronium ion concentration.
Answers: 1


Chemistry, 22.06.2019 06:00
The tilt of the earth's axis of rotation is responsible for the a) ocean's tides. b) size of the moon. c) brightness of stars. d) earth’s seasons.
Answers: 1
You know the right answer?
Calculate the heat released when 5.00 l of cl2(g) with a density of 1.88 g/l reacts with an excess o...
Questions


English, 31.03.2020 23:19



History, 31.03.2020 23:19


Health, 31.03.2020 23:19

English, 31.03.2020 23:19

Mathematics, 31.03.2020 23:19

Mathematics, 31.03.2020 23:19

History, 31.03.2020 23:19

Mathematics, 31.03.2020 23:19

Biology, 31.03.2020 23:19

History, 31.03.2020 23:19

Mathematics, 31.03.2020 23:19


Physics, 31.03.2020 23:19



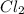 which has density of 1.88 g
which has density of 1.88 g

