
Chemistry, 16.07.2019 02:00 mickellife4659
Ineed half reactions, for the following equation. ba + znso4 -> baso4 + zn i need a oxidation side and a reduction side i will be giving brainlist to the first correct answer, do not give me answers from the web, trust me i have already tried

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 12:00
What is the percentage of hydrogen in nitrogen trihydride
Answers: 1

Chemistry, 22.06.2019 14:00
Ascientist measures the speed of sound in a monatomic gas to be 449 m/s at 20∘c. what is the molar mass of this gas?
Answers: 2

Chemistry, 22.06.2019 14:30
Is a pencil falling to the floor anon contact force, a force, or a contact force
Answers: 1

Chemistry, 22.06.2019 14:50
Consider the following multistep reaction: a b→ab(slow) a ab→a2b(fast)−−−−−−−−−−−−−−−−− 2a b→a2b(overall) based on this mechanism, determine the rate law for the overall reaction. express your answer in standard masteringchemistry format. for example, if the rate law is k[a]3[b]2 type k*[a]^3*[b]^2
Answers: 3
You know the right answer?
Ineed half reactions, for the following equation. ba + znso4 -> baso4 + zn i need a oxidation si...
Questions

Mathematics, 09.10.2019 07:30


Mathematics, 09.10.2019 07:30

English, 09.10.2019 07:30

Mathematics, 09.10.2019 07:30

History, 09.10.2019 07:30

Mathematics, 09.10.2019 07:30

Mathematics, 09.10.2019 07:30


Mathematics, 09.10.2019 07:30

Mathematics, 09.10.2019 07:30


Computers and Technology, 09.10.2019 07:30

Biology, 09.10.2019 07:30

Mathematics, 09.10.2019 07:30

Mathematics, 09.10.2019 07:30


Chemistry, 09.10.2019 07:30


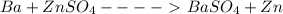
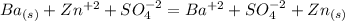
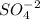 will get cancelled.
will get cancelled.
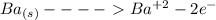 ...this is oxidation reaction as Ba is getting reduced to
...this is oxidation reaction as Ba is getting reduced to 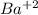 by losing 2 electrons.
by losing 2 electrons.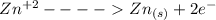 ...this is reduction reaction as
...this is reduction reaction as 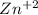 is getting reduced to Zn by gaining 2 electrons.
is getting reduced to Zn by gaining 2 electrons.


