
Chemistry, 16.07.2019 04:00 cmfield8810
Hno3, a strong acid, is added to shift the ag2co3 equilibrium (equation 7.6) to the right. explain why the shift occurs. b. what would have been observed if hcl (also a strong acid) had been added instead of the hno3?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 09:00
Suppose you have designed a new thermometer called the x thermometer. on the x scale the boiling point of water is 129 ? x and the freezing point of water is 13 ? x. part a at what temperature are the readings on the fahrenheit and x thermometers the same?
Answers: 1



Chemistry, 23.06.2019 12:30
Idid a lab for chemistry where we put nails in a copper (ii) chloride solution. 1. why did the reaction stop? which reactant was used up? how do you know? 2. describe what was happening to the atoms of iron and copper during the reaction. what is this type of reaction called? 3. what would happen to the ratio of copper to iron if you had placed more nails in the beaker? if you had let the reaction go for less time? 4. what is the accepted ratio of copper atoms to iron atoms in this reaction? account for differences between your experimental value and the accepted value. write the balanced equation for the reaction.
Answers: 2
You know the right answer?
Hno3, a strong acid, is added to shift the ag2co3 equilibrium (equation 7.6) to the right. explain w...
Questions


History, 12.03.2021 01:00

Mathematics, 12.03.2021 01:00



Mathematics, 12.03.2021 01:00

Mathematics, 12.03.2021 01:00


Mathematics, 12.03.2021 01:00



History, 12.03.2021 01:00


History, 12.03.2021 01:00

History, 12.03.2021 01:00

Mathematics, 12.03.2021 01:00

Medicine, 12.03.2021 01:00

Mathematics, 12.03.2021 01:00


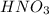 reacts with
reacts with 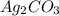 usually the equilibrium shifts to the right because
usually the equilibrium shifts to the right because 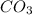 molecule from the reaction mixture, which then tends to shift the equilibrium towards right.
molecule from the reaction mixture, which then tends to shift the equilibrium towards right.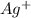 and
and 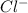 ions from the solution.
ions from the solution.

