
Chemistry, 16.07.2019 07:00 Arellano4672
For an exothermic dissolving process, predict the signs for the change in enthalpy, entropy, and free energy and explain how temperature will affect these changes.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 23:30
Start an single atom tab. observe the decay of polonium-211. after each decay, press the reset nucleus button to watch the process again. write a description of alpha decay for po-211
Answers: 2

Chemistry, 22.06.2019 10:10
Stage in which a typical star has completely stopped fusion
Answers: 1

Chemistry, 22.06.2019 16:00
How could a student test the effect of removing heat from a gas that is stored in a sealed container? what must occur in order for matter to change states?
Answers: 2

Chemistry, 23.06.2019 04:31
2ki + pb(no3)2 → 2kno3 + pbi2 determine how many moles of kno3 are created if 0.03 moles of ki are completely consumed.
Answers: 1
You know the right answer?
For an exothermic dissolving process, predict the signs for the change in enthalpy, entropy, and fre...
Questions




Chemistry, 06.04.2021 18:50

History, 06.04.2021 18:50

Health, 06.04.2021 18:50


Health, 06.04.2021 18:50

English, 06.04.2021 18:50

History, 06.04.2021 18:50

Mathematics, 06.04.2021 18:50

SAT, 06.04.2021 18:50





Mathematics, 06.04.2021 18:50


History, 06.04.2021 18:50

History, 06.04.2021 18:50

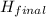 -
- 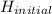 (
(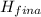 <
< 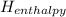 should always be negative.
should always be negative.

