
Chemistry, 16.07.2019 08:00 athenadailey8717
If 30.0 ml of 0.150 m aqueous sodium hydroxide is mixed with 30.0 ml of 0.150 m aqueous hydrochloric acid in a calorimeter at an initial temperature of 25.0 degrees celsius, what is the enthalpy change of this reaction if the final temperature reached in the calorimeter is 27.5 degrees celsius? naoh + hcl nacl + h2o

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 14:00
The two naturally occurring isotopes of chlorine are 35cl (34.969 amu, 75.77%) and 37cl (36.966 amu, 24.23%). the two naturally occurring isotopes of bromine are 79br (78.918 rm amu, 50.69%) and 81br (80.916 amu, 49.31%). chlorine and bromine combine to form bromine monochloride, brcl. 1. how many peaks will be present in a mass spectrum for brcl? the four combinations of molecule possible given these four isotopes are: 81br37cl, 81br35cl, 79br37cl, and 79br35cl. 2. what are the masses of the four different brcl molecules? express the masses using six significant figures, in decreasing numeric order (highest to lowest), separated by commas.
Answers: 3


Chemistry, 22.06.2019 23:00
What element has similar physical and chemical properties as boron.
Answers: 1

Chemistry, 23.06.2019 06:30
The velocity of any object depends upon a) the location of the object. b) the location of the observer. c) which measurement tools are used. d) the relative motion of the observer.
Answers: 1
You know the right answer?
If 30.0 ml of 0.150 m aqueous sodium hydroxide is mixed with 30.0 ml of 0.150 m aqueous hydrochloric...
Questions






Mathematics, 23.06.2019 11:50

Computers and Technology, 23.06.2019 11:50

Mathematics, 23.06.2019 11:50

Advanced Placement (AP), 23.06.2019 11:50


Mathematics, 23.06.2019 11:50



Health, 23.06.2019 11:50


Mathematics, 23.06.2019 11:50



Advanced Placement (AP), 23.06.2019 11:50


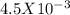 moles.
moles.

