

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 20:00
The volume of a single vanadium atom is 9.29×10-24 cm3. what is the volume of a vanadium atom in microliters?
Answers: 3

Chemistry, 22.06.2019 22:00
The volume of an unknown substance in a sealed glass jar is 50 milliliters. the volume of the jar is 200 milliliters. which state of matter could the substance be?
Answers: 2

Chemistry, 23.06.2019 00:00
(04.05 hc) analyze the given diagram of the carbon cycle below. part 1: which compound does c represent? part 2: name a process that could release this compound into the air. part 3: explain how the elements that form it are conserved during the carbon cycle. use complete sentences to explain your answer. justify how this compound was created from a recycling of carbon in the carbon cycle. use complete sentences to explain your answer.
Answers: 3
You know the right answer?
When iron rusts in air iron3 oxide is produced. how many moles of oxygen react with 28.0 mol of iron...
Questions

Computers and Technology, 16.09.2019 05:30

English, 16.09.2019 05:30

Mathematics, 16.09.2019 05:30


Biology, 16.09.2019 05:30

Social Studies, 16.09.2019 05:30



Mathematics, 16.09.2019 05:30



Mathematics, 16.09.2019 05:30




Chemistry, 16.09.2019 05:30


Social Studies, 16.09.2019 05:30

Chemistry, 16.09.2019 05:30

Chemistry, 16.09.2019 05:30

 .
.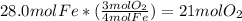
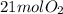 is produced.
is produced.


