
Chemistry, 17.07.2019 00:00 Bryson2148
Which of the following are chemical properties of baking soda? choose all that are correct. select one or more: a. releases co2 when heated. b. reacts vigorously with acids. c. generally found as a white powder. d. readily dissolves in water. the state of matter in which a material has a definite volume but no definite shape is the select one: a. gaseous state. b. solid state. c. frozen state. d. liquid state. to illustrate the arrangement of particles in a liquid, a teacher instructed 20 students to get up and move to a random spot in the classroom. what should the teacher tell the students to do to illustrate the arrangement of particles in a solid? select one: a. find the person who is closest and stand next to him or her. b. choose a new random spot, this time outside of the classroom. c. go to the center of the room and create five rows of four students. d. form four separate groups of different sizes and go to the corners.
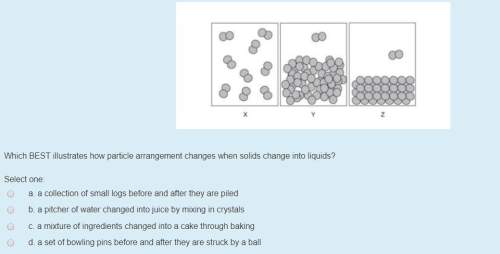

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 14:30
According to le chatelier’s principle, a system in chemical equilibrium responds to stress by shifting the equilibrium in a direction that reduces the stress. normalizes the stress. increases the stress. changes the stress.
Answers: 1

Chemistry, 22.06.2019 20:00
In vapor-liquid equilibrium in a binary mixture, both components are generally present in both phases. how many degrees of freedom are there for such a system? the reaction between nitrogen and hydrogen to form ammonia occurs in the gas phase. how many degrees of freedom are there for this system? steam and coal react at high temperatures to form hydrogen, carbon monoxide, carbon dioxide, and methane. the following reactions have been suggested as being involved in the chemical transformation:
Answers: 3

Chemistry, 22.06.2019 20:10
The lattice enthalpy (formation of ionic solid from ions in the gas phase) for agcl(s) is -916 kj/mol and the hydration enthalpy (dissolution of gaseous ions into water) is -850 kj/mol. how much heat (in joules) is involved in forming 1l of saturated agcl solution (1.8 × 10-4 g / 100 ml water) by dissolving agcl(s)? assume solution volume does not change much upon dissolution. the equations are given below. ag+(g) + cl−(g) æ agcl(s)
Answers: 3

Chemistry, 22.06.2019 22:30
The vapor pressure of ethanol is 1.00 × 102 mmhg at 34.90°c. what is its vapor pressure at 61.61°c? (δhvap for ethanol is 39.3 kj/mol.)
Answers: 2
You know the right answer?
Which of the following are chemical properties of baking soda? choose all that are correct. select...
Questions

Mathematics, 09.06.2021 21:10



English, 09.06.2021 21:10



English, 09.06.2021 21:10


Chemistry, 09.06.2021 21:10




Mathematics, 09.06.2021 21:10

Mathematics, 09.06.2021 21:10



Mathematics, 09.06.2021 21:10

Mathematics, 09.06.2021 21:10





