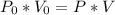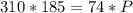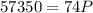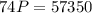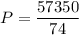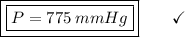Chemistry, 17.07.2019 07:00 jesuslovesusall3
Acontainer filled with gas has a volume of 185ml and a pressure of 310mm hg. the desired new volume is 74.0 ml. what is the required new pressure? 124 mm hg 44 mm hg 775 mm hg 0.00129 mm hg

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 08:00
What is the molarity of 60.0 grams of naoh dissolved in 750 milliliters of water? a) 1.1 m b) 2.0 m c) 12 m d) 75 m
Answers: 1

Chemistry, 22.06.2019 08:30
If i initially have a gas at a pressure of 12 atm, a volume of 23 liters, and a temperature of 200 k, and then i raise the pressure to 14 atm and increase the temperature to 300 k, what is the new volume of the gas?
Answers: 1

Chemistry, 23.06.2019 00:30
Titration reveals that 11.6 ml of 3.0m sulfuric acid are required to neutralize the sodium hydroxide in 25.00ml of naoh solution. what is the molarity of the naoh solution?
Answers: 1

You know the right answer?
Acontainer filled with gas has a volume of 185ml and a pressure of 310mm hg. the desired new volume...
Questions


Computers and Technology, 18.10.2020 06:01


Spanish, 18.10.2020 06:01

Mathematics, 18.10.2020 06:01

Mathematics, 18.10.2020 06:01


Mathematics, 18.10.2020 06:01


Mathematics, 18.10.2020 06:01



Mathematics, 18.10.2020 06:01




Mathematics, 18.10.2020 06:01


Mathematics, 18.10.2020 06:01