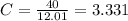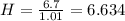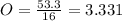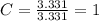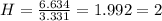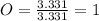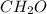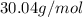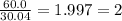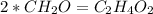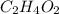Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:30
In order to calculate the amount of heat transferred you must know the __ and specific heat of the material, as well as the change in temperature. a. volume b. density c. mass d. enthalpy
Answers: 1

Chemistry, 22.06.2019 03:30
If a solution is considered basic, then a) the hydroxide ion and hydronium ion concentrations are equal. b) the hydroxide ion concentration is less than the hydronium ion concentration. c) the hydronium ion concentration is greater than the hydroxide ion concentration. d) the hydroxide ion concentration is greater than the hydronium ion concentration.
Answers: 1

Chemistry, 22.06.2019 07:10
Provide a stepwise curved arrow mechanism that fully explains the outcome of the reaction shown below. oh нао* heat он
Answers: 2

Chemistry, 22.06.2019 12:10
Achemistry student needs to standardize a fresh solution of sodium hydroxide. he carefully weighs out of oxalic acid , a diprotic acid that can be purchased inexpensively in high purity, and dissolves it in of distilled water. the student then titrates the oxalic acid solution with his sodium hydroxide solution. when the titration reaches the equivalence point, the student finds he has used of sodium hydroxide solution.calculate the molarity of the student's sodium hydroxide solution. be sure your answer has the correct number of significant digits.
Answers: 1
You know the right answer?
An unknown compound is found to contain 40.0 percent carbon, 6.7 percent hydrogen and 53.3 percent o...
Questions

English, 15.03.2020 21:26

Mathematics, 15.03.2020 21:26

History, 15.03.2020 21:27

Mathematics, 15.03.2020 21:27

Biology, 15.03.2020 21:27

Mathematics, 15.03.2020 21:27


Chemistry, 15.03.2020 21:27

Mathematics, 15.03.2020 21:28


Mathematics, 15.03.2020 21:29

Mathematics, 15.03.2020 21:29

Mathematics, 15.03.2020 21:30

Physics, 15.03.2020 21:32


English, 15.03.2020 21:34


Social Studies, 15.03.2020 21:36


Chemistry, 15.03.2020 21:38