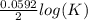Chemistry, 17.07.2019 08:00 crawford184232323234
What is the value of the equilibrium constant for this redox reaction? 2ag+(aq) + zn (s) → 2ag (s) + zn2+(aq) e = +1.56 v k=10(ne∘0.0592) k = 2.25 × 10-26 k = 2.25 × 1026 k = 5.04 × 10-52 k = 5.04 × 1052

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Consider the following system at equilibrium. caco3(s) ca2+(aq) + co32–(aq) the addition of which compound will cause a shift in equilibrium because of a common ion effect? ccl4 co2 cuso4 na2co3
Answers: 3

Chemistry, 22.06.2019 16:10
Predict the reactants of this chemical reaction. that is, fill in the left side of the chemical equation. be sure the equation you submit is balanced. (you can edit both sides of the equation to balance it, if you need to.) note: you are writing the molecular, and not the net ionic equation. > cacl2(aq) + h20(l)
Answers: 2

Chemistry, 22.06.2019 20:00
Which of the following would not diffuse through the plasma membrane by means of simple diffusion? 1 oxygen 2 glucose 3 a steroid hormone 4 a lipid soluble vitamin
Answers: 3

Chemistry, 22.06.2019 22:30
Essay-alternative energy sources research sources of energy that are being developed. write a report of 350-400 words discussing the information you learned concerning the development of various energy sources and the impact that you think they will have on your life. include sources cited at the end of your report using the mla format. follow the rubric guidelines. note that wikipedia is not an appropriate resource for a research paper. worth 99
Answers: 3
You know the right answer?
What is the value of the equilibrium constant for this redox reaction? 2ag+(aq) + zn (s) → 2ag (s)...
Questions

Mathematics, 26.10.2020 19:30



Mathematics, 26.10.2020 19:30

Mathematics, 26.10.2020 19:30

Mathematics, 26.10.2020 19:30

History, 26.10.2020 19:30

English, 26.10.2020 19:30







Physics, 26.10.2020 19:30



Mathematics, 26.10.2020 19:30


 ,
,