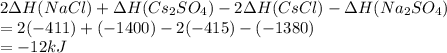Chemistry, 17.07.2019 08:30 ayoismeisalex
Which of the following displays the correct change in enthalpy and best describes the reaction below? 2cscl(aq) + na2so4(aq) 2nacl(aq) + cs2so4(aq) given: cs2so4: ∆h= -1400 kj cscl: ∆h= -415 kj na2so4: ∆h= -1380 kj nacl: ∆h = -411 kj

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:00
What three natural resources are found in the great lakes region
Answers: 2

Chemistry, 22.06.2019 14:30
100 grams of molten lead (600°c) is used to make musket balls. if the lead shot is allowed to cool to room temperature (21°c), what is the change in entropy (in j/k) of the lead? (for the specific heat of molten and solid lead use 1.29 j/g⋅°c; the latent heat of fusion and the melting point of lead are 2.45 × 104 j/kg and 327°c, respectively.)
Answers: 1

Chemistry, 22.06.2019 20:00
How are the terms group and period used on the periodic table
Answers: 1

Chemistry, 22.06.2019 23:30
Rank substituents in order of their priority when assigning the e or z label to an alkene. i, ch2i , h, ch2ch2cl, f
Answers: 2
You know the right answer?
Which of the following displays the correct change in enthalpy and best describes the reaction below...
Questions





Geography, 16.12.2019 23:31

Computers and Technology, 16.12.2019 23:31

Mathematics, 16.12.2019 23:31


Mathematics, 16.12.2019 23:31




Computers and Technology, 16.12.2019 23:31


Arts, 16.12.2019 23:31



Mathematics, 16.12.2019 23:31