From the diagram, when the temperatures of reactants are raised from 20°c to 30°c:
a. the ave...

Chemistry, 09.10.2019 19:30 estefaniapenalo
From the diagram, when the temperatures of reactants are raised from 20°c to 30°c:
a. the average kinetic energy doubles.
b. the number of molecules doubles.
c. the total number of collisions doubles.
d. the number of molecules having er or greater doubles.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Using the periodic table, complete the table to describe each atom. type in your answers.a ? b? c? d? e? f?
Answers: 1

Chemistry, 22.06.2019 09:10
When a nucleus absorbs a neutron and then breaks apart, there are many products of the reaction. what is not a product of a nuclear fission reaction
Answers: 1

Chemistry, 23.06.2019 01:00
Who examines and coordinates the cleanup of polluted sites?
Answers: 2

Chemistry, 23.06.2019 08:00
Pl what kind of reaction is this? nahco3 + h2o → co2 + naoh + h2o -composition -decomposition -single replacement -double replacement im leaning more toward single replacement. if im wrong can you explain whyy?
Answers: 2
You know the right answer?
Questions


Arts, 11.05.2021 18:20

Health, 11.05.2021 18:20


Mathematics, 11.05.2021 18:20

English, 11.05.2021 18:20


Computers and Technology, 11.05.2021 18:20

Arts, 11.05.2021 18:20



Mathematics, 11.05.2021 18:20


Mathematics, 11.05.2021 18:20


Mathematics, 11.05.2021 18:20

Mathematics, 11.05.2021 18:20

History, 11.05.2021 18:20


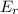 which means now double number of molecules will be able to cross the energy barrier and hence the formation of product increases and hence the rate increases to double.
which means now double number of molecules will be able to cross the energy barrier and hence the formation of product increases and hence the rate increases to double.

