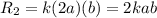Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 14:30
Find the mass in grams of hydrogen gas produced when 14.0 moles of hcl is added to an excess amount of magnesium.
Answers: 3

Chemistry, 21.06.2019 23:30
What’s the scientific notation for the number 6,840,000,000
Answers: 1

Chemistry, 22.06.2019 08:30
What method(s) do plants use to obtain nitrogen? select all that apply. absorb it from the atmosphere use bacteria to convert nitrogen to usable form obtain usable nitrogen compounds from the soil absorb nitrogen from water taken in at the roots
Answers: 3

Chemistry, 22.06.2019 12:30
Place the elements below in order of decreasing ionization energy. aluminum(al) chlorine(cl) magnesium (mg) sulfur(s)
Answers: 1
You know the right answer?
Consider two gases, a and b, are in a container at room temperature. what effect will the following...
Questions

Computers and Technology, 12.12.2020 16:20

Mathematics, 12.12.2020 16:20



Mathematics, 12.12.2020 16:20






Social Studies, 12.12.2020 16:20

History, 12.12.2020 16:20

Social Studies, 12.12.2020 16:20

Mathematics, 12.12.2020 16:20

English, 12.12.2020 16:20


Mathematics, 12.12.2020 16:20

English, 12.12.2020 16:20


Mathematics, 12.12.2020 16:20

 product
product![R_1=k[A][B]=kab](/tpl/images/0213/1347/57084.png)