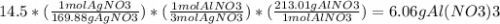Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 05:30
The table describes how some substances were formed substance 19 description formed by boiling pure water formed by combining three hydrogen atoms to every nitrogen atom formed by adding 5 g of sugar to 1 l of water formed by compressing carbon under high pressure based on the given descriptions, which substance is most likely a mixture?
Answers: 1

Chemistry, 22.06.2019 10:30
Astudent reacts 13 moles of iron with 21 moles of oxygen according to the following equation:
Answers: 1

Chemistry, 22.06.2019 16:50
Answer asap need by wednesday morning calculate the ph of 0.16m ch3cooh which has ka = 1.74 x 10-5 mol dm-3 best answer will be brainliest
Answers: 3

Chemistry, 23.06.2019 04:40
Equal numbers of moles of he(g), ar(g), and ne(g) are placed in a glass vessel at room temperature. if the vessel has a pinhole-sized leak, which of the following will be true regarding the relative values of the partial pressures of the gases remaining in the vessel after some of the gas mixture has effused?
Answers: 1
You know the right answer?
If 14.5 grams of silver nitrate (molar mass =169.88g/mol) reacts with aluminum chloride. how many gr...
Questions

Biology, 12.11.2020 03:20


English, 12.11.2020 03:20



History, 12.11.2020 03:20

Mathematics, 12.11.2020 03:20

Mathematics, 12.11.2020 03:20

Mathematics, 12.11.2020 03:20

Mathematics, 12.11.2020 03:20

Mathematics, 12.11.2020 03:20


English, 12.11.2020 03:20

Business, 12.11.2020 03:20

Mathematics, 12.11.2020 03:20


Spanish, 12.11.2020 03:20

Mathematics, 12.11.2020 03:20

English, 12.11.2020 03:20

Social Studies, 12.11.2020 03:20