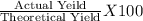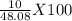Chemistry, 18.07.2019 00:30 njones58emailtjcedu
Hcl + naoh → nacl + h2o 1. if 30g of hcl is reacted with excess naoh, and 10g of nacl is produced, what is the theoretical yield of the experiment? 2. if 30 g of hcl is reacted with excess naoh, and 10 g of nacl is produced, what is the percent yield of the experiment?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 15:30
Count the number of each type of atom in the equation below, and then balance the equation. write in the numbers of atoms and coefficients. add a 1 if there should be no coefficient. cs2(l) + o2(g) → co2(g) + so2(g) c [ ] s [ ] o > c [ ] s [ ] o [ ] cs2(l) + [ ] o2(g) > [ ] co2(g) + [ ] so2(g)
Answers: 3

Chemistry, 23.06.2019 01:00
Iron (fe) reacts with copper sulfate (cuso4) to form iron (ii) sulfate. in this reaction, cu2+ gains electrons to form cu. which statement is true about this reaction? fe(s) + cuso4(aq) → feso4(aq) + cu(s)
Answers: 3

Chemistry, 23.06.2019 01:30
Which is an example of a highly unstable isotope that is often used in fission reactions?
Answers: 1

Chemistry, 23.06.2019 04:00
What are the names of these two interactions with cattle and how do they differ from each other
Answers: 3
You know the right answer?
Hcl + naoh → nacl + h2o 1. if 30g of hcl is reacted with excess naoh, and 10g of nacl is produced, w...
Questions

Mathematics, 17.03.2021 23:40

Mathematics, 17.03.2021 23:40


Chemistry, 17.03.2021 23:40



English, 17.03.2021 23:40





Mathematics, 17.03.2021 23:40

Geography, 17.03.2021 23:40


Computers and Technology, 17.03.2021 23:40

English, 17.03.2021 23:40



History, 17.03.2021 23:40