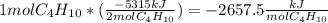Butane (c4 h10(g), mc031-1.jpghf = –125.6 kj/mol) reacts with oxygen to produce carbon dioxide (co2 , mc031-2.jpghf = –393.5 kj/mol ) and water (h2 o, mc031-3.jpghf = –241.82 kj/mol) according to the equation below. mc031-4.jpg what is the enthalpy of combustion (per mole) of c4h10 (g)? use mc031-5.jpg. –2,657.5 kj/mol –5315.0 kj/mol –509.7 kj/mol –254.8 kj/mol

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 15:00
If a planet rotates 360 degrees during a 24 hour time period, what does that tell us about the planet? a. the middle of the planet is in darkness b. the seasons on the planet vary every day. c. the planet runs on a 12-hour time clock. d. the temperature on the planet varies daily.
Answers: 1

Chemistry, 22.06.2019 12:30
The missing component to the table to the right or indicated with orange letters complete the table by filling in the corresponding numbers or symbols
Answers: 3

Chemistry, 23.06.2019 09:00
Chortling is used to clean water. another possible atom that would also work is a. sodium b. sulfur c. bromine
Answers: 1

Chemistry, 23.06.2019 10:00
1.9 mol hcl and 3.9 mol naoh react according to the equation hcl + naoh −→ nacl + h2o . if the limiting reactant is hcl, calculate the amount of nacl formed.
Answers: 1
You know the right answer?
Butane (c4 h10(g), mc031-1.jpghf = –125.6 kj/mol) reacts with oxygen to produce carbon dioxide (co2...
Questions

Mathematics, 13.12.2019 20:31


Mathematics, 13.12.2019 20:31


History, 13.12.2019 20:31

English, 13.12.2019 20:31


Mathematics, 13.12.2019 20:31





Health, 13.12.2019 20:31





Mathematics, 13.12.2019 20:31

Mathematics, 13.12.2019 20:31

Mathematics, 13.12.2019 20:31

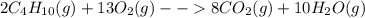
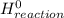 = Σ
= Σ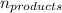 Δ
Δ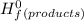 -Σ
-Σ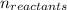 Δ
Δ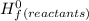
![[{8*(-393.5kJ/mol)}+{10*(-241.82kJ/mol)}]-[{2*(-125.6kJ/mol)}+13*(0 kJ/mol)}]](/tpl/images/0106/1493/0d4a2.png) =[-3148kJ/mol+(-2418.2kJ/mol)]-[(-251.2kJ/mol)+0]
=[-3148kJ/mol+(-2418.2kJ/mol)]-[(-251.2kJ/mol)+0]