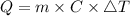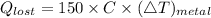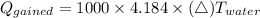If you drop a 150-gram piece of metal that has a temperature of 125° celsius into 1000 grams of water at 20° celsius, what best describes what would occur? (2 points) more heat will be lost by the metal than gained by the water. the temperature of the water will be greater than the amount of heat lost by the metal. the temperature change of the metal will equal the temperature change of the water. the heat lost by the metal will equal the heat gained by the water.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 05:30
Arecipe calls for 1.2 cups of oil. how many liters of oil is this?
Answers: 2

Chemistry, 22.06.2019 10:30
When the speed of the bottle is 2 m/s, the average maximum height of the beanbag is m.
Answers: 2

Chemistry, 22.06.2019 18:20
Categorize them by metal, nonmetal, in periodic tableductilenon-ductilemalleableoften gain electrons easilygood conductorpoor conductorcan be liquidselements
Answers: 2

Chemistry, 22.06.2019 19:30
Astring vibrates with a frequency of 10 hz. why can't a person hear the sound waves produced by the vibrating string, no matter how large the amplitude of the waves? out! this is homework and due tomorrow! you so much!
Answers: 2
You know the right answer?
If you drop a 150-gram piece of metal that has a temperature of 125° celsius into 1000 grams of wate...
Questions

Arts, 28.01.2020 06:31

Biology, 28.01.2020 06:31


Biology, 28.01.2020 06:31







Mathematics, 28.01.2020 06:31

Mathematics, 28.01.2020 06:31



English, 28.01.2020 06:31

Business, 28.01.2020 06:31

Biology, 28.01.2020 06:31

Mathematics, 28.01.2020 06:31

Social Studies, 28.01.2020 06:31

Mathematics, 28.01.2020 06:31