Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 02:20
Compared with the freezing-point depression of a 0.01 m c6h12o6 solution, the freezing-point depression of a 0.01 m nacl solution is
Answers: 1


Chemistry, 23.06.2019 16:30
All chemical reactions use reactants in a specific proportion or stoichiometry to form products. the reactant regulates the amount of products produced. a) excess b) limiting c) proportional d) stoichiometric
Answers: 1

You know the right answer?
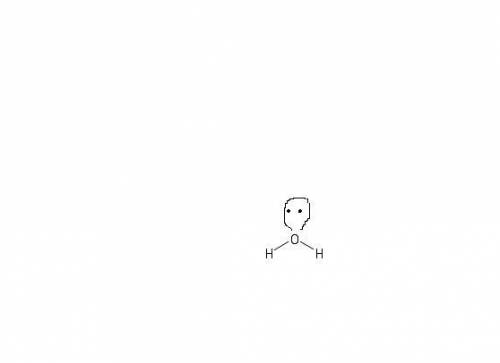
How does a lone pair contribute to molecular shape? a. it is too small to affect the molecule's sha...
Questions

History, 21.06.2019 15:30


Chemistry, 21.06.2019 15:30


Geography, 21.06.2019 15:30

Mathematics, 21.06.2019 15:30

Chemistry, 21.06.2019 15:30




Chemistry, 21.06.2019 15:30

Health, 21.06.2019 15:30




English, 21.06.2019 15:30

Chemistry, 21.06.2019 15:30

Physics, 21.06.2019 15:30