
Chemistry, 19.07.2019 06:00 Jaedenaleinson
Calculate the freezing point of a solution made from 52.6 g of propane, c3h8, dissolved in 196.0 g of benzene, c6h6. the freezing point of benzene is 5.50 c and its kf is 5.12 c/m.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 02:30
Margaret wants to make an orange flavored drink by stirring powdered drink mix into a glass of water. she doesn't like drinks that have small clumps of powdered solid in them, so she wants the drink to be a perfect solution. what factors should margaret not consider when deciding how much powder to add to her glass of water?
Answers: 3

Chemistry, 22.06.2019 11:00
The human eye contains a molecule called 11-cis-retinal that changes shape when struck with light of sufficient energy. the change in shape triggers a series of events that results in an electrical signal being sent to the brain that results in vision. the minimum energy required to change the conformation of 11-cis-retinal within the eye is about 164 kj/mol.
Answers: 2

Chemistry, 22.06.2019 19:30
What is the area in square meters of 448 g ai foil that has a thickness of 23921 nm? the density is 2.70 g/cm
Answers: 3

You know the right answer?
Calculate the freezing point of a solution made from 52.6 g of propane, c3h8, dissolved in 196.0 g o...
Questions


Mathematics, 29.04.2021 15:40


Mathematics, 29.04.2021 15:40

Mathematics, 29.04.2021 15:40

Social Studies, 29.04.2021 15:40

Mathematics, 29.04.2021 15:40




English, 29.04.2021 15:40



Mathematics, 29.04.2021 15:40

Mathematics, 29.04.2021 15:40

Biology, 29.04.2021 15:40



Mathematics, 29.04.2021 15:40

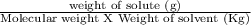
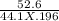 = 6.085 m
= 6.085 m

