
Chemistry, 19.07.2019 08:30 sabrinarasull1pe6s61
Assume you have 60 micrograms (1 microgram = 10-6 gram) of pure u-238. how many nuclei n0 is this? (recall avogadro's number equals 6.022×1023 particles per mole.)

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 06:00
In an investigation that uses the scientific method, which step immediately follows making a hypothesis? o summarizing the results o asking a question o making observations designing an experiment mark this and retum save and exit next submit
Answers: 2

Chemistry, 22.06.2019 10:30
Use this information to determine the number of calends electrons in the atoms. which of the following correctly compares the stability of the two atoms? a) both are unreactive b) both are highly reactive c) a is unreactive and d is reactive d) a is reactive and d is unreactive
Answers: 2

Chemistry, 22.06.2019 12:20
Achemistry student weighs out 0.306 g of citric acid (h3c6h5o7), a triprotic acid, into a 250 ml volumetric flask and dilutes to the mark with distilled water. he plans to titrate the acid with 0.1000 m naoh solution. calculate the volume of naoh solution the student will need to add to reach the final equivalence point. be sure your answer has the correct number of significant digits.
Answers: 3
You know the right answer?
Assume you have 60 micrograms (1 microgram = 10-6 gram) of pure u-238. how many nuclei n0 is this?...
Questions



English, 04.09.2019 16:30

Mathematics, 04.09.2019 16:30

Physics, 04.09.2019 16:30





Advanced Placement (AP), 04.09.2019 16:30



English, 04.09.2019 16:30





Biology, 04.09.2019 16:30

English, 04.09.2019 16:30

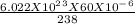 = 1.518 X 10^17 nuclei of Uranium.
= 1.518 X 10^17 nuclei of Uranium.

