
1. a scientist studies the reaction 2no2(g) 2no(g) + o2(g). she performs three experiments using different concentrations of no2 and measures the initial reaction rate. experiment : [no2] (mol/l) : initial rate ((mol/l)/s) 1 : 0.1 : 0.006 2 : 0.3 : 0.054 3 : 0.5 : 0.150 a. what is the ratio of the concentrations between trials 1 and 2? (2 points) b. what is the ratio of the initial reaction rates between trials 1 and 2? (2 points) c. what is the exponent for [no2] in the rate law? (2 points) d. write the rate law. (2 points) e. solve for the value of k. (2 points) f. what is the overall reaction order? (2 points)

Answers: 1


Another question on Chemistry


Chemistry, 21.06.2019 18:30
In a sample of oxygen gas at room temperature, the average kinetic energy of all the balls stays constant. which postulate of kinetic molecular theory best explains how this is possible?
Answers: 2

Chemistry, 21.06.2019 22:00
To save time, you can approximate the initial mass of the solid to the nearest ±1 g. for example, if you are asked to add 14.3 g of copper, add between 13 g and 15 g. which of the following sets include two samples with an equal density? which all that apply below 15.4 g gold and 18.7 g silver 15.2 g copper and 50.0 g copper 20.2 g silver and 20.2 g copper 11.2 g gold and 14.9 g gold
Answers: 1

Chemistry, 22.06.2019 06:30
(1.6 × 10-19)(5.0 × 106) = c × 10d identify the missing numbers below to show the result of multiplying the numbers.
Answers: 1
You know the right answer?
1. a scientist studies the reaction 2no2(g) 2no(g) + o2(g). she performs three experiments using dif...
Questions






Mathematics, 17.09.2019 04:00



Biology, 17.09.2019 04:00

History, 17.09.2019 04:00

Health, 17.09.2019 04:00

Mathematics, 17.09.2019 04:00








Mathematics, 17.09.2019 04:00

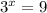 , which gives x = 2, and the exponent in the rate law is 2.
, which gives x = 2, and the exponent in the rate law is 2.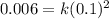 , which yields k = 0.6 L/mol-s.
, which yields k = 0.6 L/mol-s.

