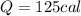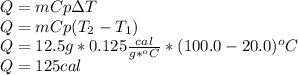Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 17:10
The concept of empiricism states that all rationally accepted knowledge is determined from experience. francis bacon was one of the first scientists to promote this theory. what was it’s impact on society?
Answers: 1

Chemistry, 22.06.2019 05:50
What are the 4 phases of matter in order of increasing engery content?
Answers: 2

Chemistry, 22.06.2019 14:50
Given the following information: mass of proton = 1.00728 amu mass of neutron = 1.00866 amu mass of electron = 5.486 × 10^-4 amu speed of light = 2.9979 × 10^8 m/s calculate the nuclear binding energy (absolute value) of 3li^6. which has an atomic mass of 6.015126 amu. j/mol.
Answers: 2

Chemistry, 22.06.2019 15:10
Which statement describes the phase change that occurs when dry ice is placed in an open container at room temperature?
Answers: 1
You know the right answer?
The specific heat of aluminum is 0.125 cal/g °c. if 12.5 grams of aluminum were heated from 20.0 °c...
Questions

English, 05.11.2020 03:10

Business, 05.11.2020 03:10

Mathematics, 05.11.2020 03:10

Chemistry, 05.11.2020 03:10

Mathematics, 05.11.2020 03:10


English, 05.11.2020 03:10


Mathematics, 05.11.2020 03:10


Mathematics, 05.11.2020 03:10





Mathematics, 05.11.2020 03:20

Social Studies, 05.11.2020 03:20