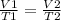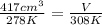Chemistry, 19.07.2019 19:30 Studyhard3332
Astudent increases the temperature of a 417cm³ balloon from 278k to 231k. assuming constant pressure, what should the new volume of the balloon be? a. 417cm³ b. 376cm³ c. 924cm³ d. 462cm³

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 13:00
If two objects at different te,peraure are in contact with each other what happens to their temperature
Answers: 1

Chemistry, 22.06.2019 20:30
Identify the correct mole ratio for each substance. sodium chloride (nacl) na: cl = 1: ammonium nitrate (nhno) h: o = 4:
Answers: 1

Chemistry, 22.06.2019 21:30
If 22.5 of nitrogen at 748 mm hg are compressed to 725 mm hg at constant temperature. what is the new volume?
Answers: 1
You know the right answer?
Astudent increases the temperature of a 417cm³ balloon from 278k to 231k. assuming constant pressure...
Questions

English, 14.12.2020 20:50



Arts, 14.12.2020 20:50

Mathematics, 14.12.2020 20:50

Social Studies, 14.12.2020 20:50



Physics, 14.12.2020 20:50




Mathematics, 14.12.2020 20:50


Mathematics, 14.12.2020 20:50

Advanced Placement (AP), 14.12.2020 20:50

Mathematics, 14.12.2020 20:50