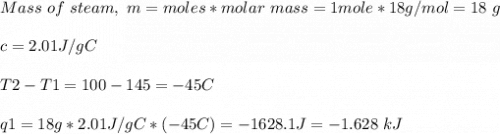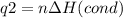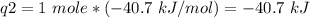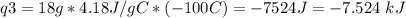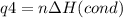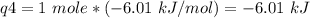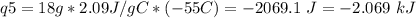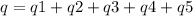Chemistry, 19.07.2019 21:00 wendelkristen
How much heat is evolved in converting 1.00 mol of steam at 145.0 ∘c to ice at -55.0 ∘c? the heat capacity of steam is 2.01 j/(g⋅∘c) and of ice is 2.09 j/(g⋅∘c).

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 07:30
In the particles are arranged in a regular, repeating pattern. a)a crystalline liquid b)a crystalline solid c)all gases d)all solids
Answers: 2

Chemistry, 22.06.2019 17:40
Which statement about hf is true? it is zero for any compound in its standard state. it is positive when the bonds of the product store more energy than those of the reactants. it is negative when a compound forms from elements in their standard states. it is zero for any element that is in the liquid state.
Answers: 1

Chemistry, 23.06.2019 06:30
Achemist is studying the following equilibirum, which has the given equilibrium constant at a certain temperature: 2 no(g) + cl2(g) < => 2 nocl(g) kp = 2 x 10^(-6)he fills a reaction vessel at this temperature with 13. atm of nitrogen monoxide gas and 12. atm of chlorine gas. use this data to answer the questions: a. can you predict the equilibrium pressure of noci, using only the tools available to you within aleks? y/nb. if you said yes, then enter the equilibrium pressure of nocl at right. round your answer to 1 significant digit.
Answers: 1

Chemistry, 23.06.2019 10:10
In a covalent bond, two atoms are held together by the attraction between . the number of covalent bonds that an atom can form depends on the number of in the atom.
Answers: 2
You know the right answer?
How much heat is evolved in converting 1.00 mol of steam at 145.0 ∘c to ice at -55.0 ∘c? the heat c...
Questions


Mathematics, 31.01.2021 14:00





Spanish, 31.01.2021 14:00

Mathematics, 31.01.2021 14:00

Chemistry, 31.01.2021 14:00

Mathematics, 31.01.2021 14:00

Chemistry, 31.01.2021 14:00

English, 31.01.2021 14:00

Mathematics, 31.01.2021 14:00






Advanced Placement (AP), 31.01.2021 14:00

Spanish, 31.01.2021 14:00