Chemistry, 20.07.2019 00:00 jeffcarpenter
Atritium nucleus is formed by combining two neutrons and a proton. the mass of this nucleus is 9.106 × 10–3 universal mass unit less than the combined mass of the particles from which it is formed. approximately how much energy is released when this nucleus is formed.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 05:00
As you watch a surfer ride a wave towards the shoreline, what is the shoreline? a) displacement reference b) reference point c) coordinate plane d) cartesian boundary
Answers: 1

Chemistry, 22.06.2019 06:00
Ethanol (c2h5oh) is produced from the fermentation of sucrose in the presence of enzymes. c12h22o11(aq) + h2o(g) 4 c2h5oh(l) + 4 co2(g) determine the theoretical yield and the percent yields of ethanol if 680. g sucrose undergoes fermentation and 326.5 g ethanol is obtained. theoretical _ g _ percent %
Answers: 1

Chemistry, 22.06.2019 18:00
How many moles of oxygen gas are produced from the decomposition of six moles of potassium chlorate
Answers: 3

Chemistry, 23.06.2019 11:00
Asolubility table shows that almost all compounds of group 1 metals are soluble in water. this general rule tells you that mgi2 is soluble rbno3 is soluble cacl2 is soluble co2 is soluble
Answers: 1
You know the right answer?
Atritium nucleus is formed by combining two neutrons and a proton. the mass of this nucleus is 9.106...
Questions

Mathematics, 28.04.2021 22:00


Mathematics, 28.04.2021 22:00

Mathematics, 28.04.2021 22:00

Mathematics, 28.04.2021 22:00

Mathematics, 28.04.2021 22:00

English, 28.04.2021 22:00



History, 28.04.2021 22:00

Mathematics, 28.04.2021 22:00

Mathematics, 28.04.2021 22:00






Mathematics, 28.04.2021 22:00


Chemistry, 28.04.2021 22:00

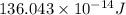
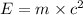
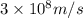
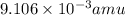
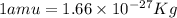
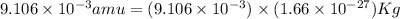
![E=[(9.106\times 10^{-3})\times (1.66\times 10^{-27})Kg]\times (3\times 10^8m/s)^2](/tpl/images/0109/7110/13e90.png)