
Chemistry, 20.07.2019 00:30 xcncxgnfxg6487
The vapor pressure of pure ethanol at 60 °c is 0.459 atm. raoult's law predicts that asolution prepared by dissolving 10.0 mmol naphthalene (nonvolatile) in 90.0 mmol ethanol willhave a vapor pressure of atm.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 19:30
If the root word engage means “to connect with something,” what does the word disengage mean in the following sentence? he disengaged the gears by stepping on the clutch pedal.a.added more engine powerb.activated a connection to the pedalc.stalled the engined.released a connection to the pedal
Answers: 1

Chemistry, 22.06.2019 13:30
Mary is conducting an experiment on how pollution affects plant growth. how can she ensure that her data are reliable?
Answers: 3

Chemistry, 22.06.2019 19:30
Anurse used a 0.02-mg/l solution of disinfection to clean a patients wound. what is the concentration of the solution expressed as a percentage?
Answers: 1

Chemistry, 22.06.2019 20:10
What would happen to a volleyball left outside in the winter? o o o o a. it would expand. b. it would lose air. c. it would shrink. d. it would explode.
Answers: 2
You know the right answer?
The vapor pressure of pure ethanol at 60 °c is 0.459 atm. raoult's law predicts that asolution prepa...
Questions



English, 20.09.2020 16:01


Health, 20.09.2020 16:01

History, 20.09.2020 16:01

Advanced Placement (AP), 20.09.2020 16:01

Chemistry, 20.09.2020 16:01




Biology, 20.09.2020 16:01

History, 20.09.2020 16:01

History, 20.09.2020 16:01






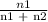 =
= 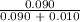 = 0.9
= 0.9

