

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 19:30
Water molecules have a strong attraction to each other because of hydrogen bonding, allowing water to move against gravity up a plant's stem through capillary action. true false
Answers: 2

Chemistry, 21.06.2019 22:00
Which of the following statements is true about planck’s law
Answers: 1

Chemistry, 22.06.2019 02:10
When 225mg of anthracene, c14h10(s), was burned in a bomb calorimeter the temperature rose by 1.75k. calculate the calorimeter constant. by how much will the temperature rise when 125mg of phenol, c6h5oh(s), is burned in the calorimeter under the same conditions? (δch< (c14h10,s)=–7061 kj mol−1.)
Answers: 3

Chemistry, 22.06.2019 18:30
Which of the following nuclei would be the least stable a 2 protons, 2 neutrons b 1 proton 1 neutron c 1 proton 3 neutrons d 1 proton 2 neutrons
Answers: 3
You know the right answer?
Cl2(g) + 2nabr(aq) 2nacl(aq) + br2(l) the reaction given above is a single replacement reaction beca...
Questions

History, 27.03.2021 08:20

English, 27.03.2021 08:20

Chemistry, 27.03.2021 08:20

Chemistry, 27.03.2021 08:20

Health, 27.03.2021 08:20

English, 27.03.2021 08:20

Mathematics, 27.03.2021 08:20

Social Studies, 27.03.2021 08:20

Mathematics, 27.03.2021 08:20

Mathematics, 27.03.2021 08:20

Mathematics, 27.03.2021 08:20



Chemistry, 27.03.2021 08:20

Advanced Placement (AP), 27.03.2021 08:20


SAT, 27.03.2021 08:20

Mathematics, 27.03.2021 08:20


Mathematics, 27.03.2021 08:20

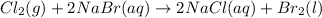
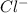 in
in 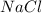 and
and 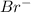 in
in 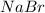 donates electrons to form
donates electrons to form 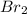 .
.


