

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 16:30
Why should the scientific method be used to answer a question? a. it provides a way to test an idea without any bias. b. it provides a way to test a hypothesis. c. it provides a way to ensure all hypotheses are proven correct. d. it provides a way to quickly turn a hypothesis into a scientific theory.
Answers: 1

Chemistry, 21.06.2019 18:00
Rutherford's experiment indicated that matter was not as uniform as it appears what part of his experimental results implied this idea
Answers: 1


Chemistry, 22.06.2019 05:00
1)each group 16 element has how many valence electrons? ( )4 ( )6 ( )8 ( )16 2)how many dots appear in the dot structure for calcium ion, ca2+? ( )zero ( )one ( )two ( )eight 3) which of the following atoms forms a cation to obtain an octet of outer shell electrons? ( )magnesium ( )oxygen ( )fluorine ( )helium 4) an al3+ ion contains 13 protons and 10 electrons. ( )true ( )false 5) valence and non-valence electrons are represented in lewis dot structures. ( )true ( )false
Answers: 3
You know the right answer?
For the reaction n2 (g) + o2 (g) 2no (g), thekeq at 2130°c is 0.0025. assume [n2] = 0.95 m, [o2] =...
Questions



Physics, 30.08.2019 16:30


Physics, 30.08.2019 16:30

Social Studies, 30.08.2019 16:30

Biology, 30.08.2019 16:30

Physics, 30.08.2019 16:30

Mathematics, 30.08.2019 16:30


History, 30.08.2019 16:30

English, 30.08.2019 16:30

History, 30.08.2019 16:30


Mathematics, 30.08.2019 16:30

History, 30.08.2019 16:30

Social Studies, 30.08.2019 16:30


History, 30.08.2019 16:30

Computers and Technology, 30.08.2019 16:30

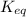
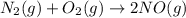
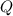 is written as:
is written as:![Q=\frac{[NO]^2}{[N_2]^1[O_2]^1}](/tpl/images/0110/0991/f0107.png)
![Q=\frac{[0.050]^2}{[0.95]^1[0.65]^1}](/tpl/images/0110/0991/2138c.png)

 , the reaction will shift towards the right i.e. towards the product side.
, the reaction will shift towards the right i.e. towards the product side.

