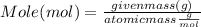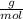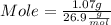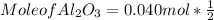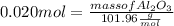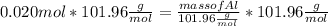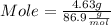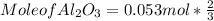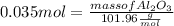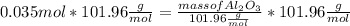Chemistry, 20.07.2019 07:30 awkwardkid0123
What mass of aluminum oxide is produced from the reaction of 4.63 g of manganese dioxide and 1.07 g of al? 3mno2 (s) + 4al(s) -> 3mn (s) + 2al2o3 (s)

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 10:30
Which characteristics can be used to differentiate star systems? check all that apply.
Answers: 2

Chemistry, 22.06.2019 14:00
In the space, show a correct numerical setup for calculating the number of moles of co2 present in 11 grams of co2
Answers: 1

Chemistry, 22.06.2019 23:10
Afusion reaction takes place between carbon and another element. neutrons are released, and a different element is formed. the different element is a) lighter than helium.b)heavier than helium.c)the same weight as helium.d)dependent on the element that reacted with carbon.
Answers: 3

Chemistry, 23.06.2019 05:00
He nucleus contains the cells genetic material in the form of dna. dna is organized into our chromosomes, which are made up of thousands of that determine our traits.
Answers: 1
You know the right answer?
What mass of aluminum oxide is produced from the reaction of 4.63 g of manganese dioxide and 1.07 g...
Questions




English, 18.12.2019 06:31

Mathematics, 18.12.2019 06:31

Chemistry, 18.12.2019 06:31

History, 18.12.2019 06:31


Mathematics, 18.12.2019 06:31

Biology, 18.12.2019 06:31




History, 18.12.2019 06:31


Mathematics, 18.12.2019 06:31



Biology, 18.12.2019 06:31