Chemistry, 20.07.2019 10:30 trinityrolle529
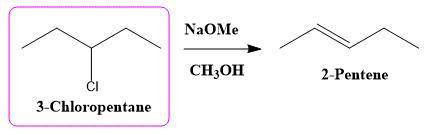
What halide would undergo dehydrohalogenation to give 2-pentene as a pure product?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 20:00
Listenbase your answer to the question on the information below.nuclear radiation is harmful to living cells, particularly to fast-growing cells, such as cancer cells and blood cells. an external beam of the radiation emitted from a radioisotope can be directed on a small area of a person to destroy cancer cells within the body.cobalt-60 is an artificially produced radioisotope that emits gamma rays and beta particles. one hospital keeps a 100.0-gram sample of cobalt-60 in an appropriate, secure storage container for future cancer treatment.which choice represents the correct product for the beta decay of the co-60? fe-60ni-60fe-61ni-61
Answers: 2

Chemistry, 22.06.2019 22:40
Percent ionization for a weak acid (ha) is determined by the following formula: percent ionization=[ha] ionized[ha] initial×100%for strong acids, ionization is nearly complete (100%) at most concentrations. however, for weak acids, the percent ionization changes significantly with concentration. the more diluted the acid is, the greater percent ionization.a certain weak acid, ha, has a ka value of 9.4×10? 7.part acalculate the percent ionization of ha in a 0.10 m solution.part bcalculate the percent ionization of ha in a 0.010 m solution
Answers: 1

Chemistry, 22.06.2019 23:30
The ammonia molecule in the diagram has the observed bond orientation because
Answers: 1

Chemistry, 23.06.2019 06:30
How can the number of core electrons be determined from the periodic table
Answers: 1
You know the right answer?
What halide would undergo dehydrohalogenation to give 2-pentene as a pure product?...
Questions


Mathematics, 25.10.2021 22:50

Computers and Technology, 25.10.2021 22:50




Chemistry, 25.10.2021 22:50


Biology, 25.10.2021 22:50

Mathematics, 25.10.2021 22:50


Chemistry, 25.10.2021 22:50

Mathematics, 25.10.2021 22:50

History, 25.10.2021 22:50

History, 25.10.2021 22:50


Mathematics, 25.10.2021 22:50



Mathematics, 25.10.2021 22:50