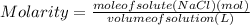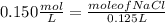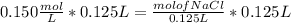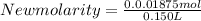Chemistry, 20.07.2019 18:00 Morganwing1019
Find the new concentration of a solution if 25.0 ml of water is added to 125.0 ml of 0.150 m nacl solution. what is the final volume? ml

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 16:30
An atom with 7 protons, 6 neutrons, and 7 electrons has an atomic mass of amu. (enter a whole number.) numerical answers expected! answer for blank 1:
Answers: 3

Chemistry, 22.06.2019 17:10
)benzene and toluene form nearly ideal solutions. consider an equimolar solution of benzene and toluene. at 20 °c the vapour pressures of pure benzene and toluene are 9.9 kpa and 2.9 kpa, respectively. the solution is boiled by reducing the external pressure below the vapour pressure. calculate (i) the pressure when boiling begins, (ii) the composition of each component in the vapour, and (iii) the vapour pressure when only a few drops of liquid remain. assume that the rate of vaporization is low enough for the temperature to remain constant at 20 °c.
Answers: 1

Chemistry, 22.06.2019 21:30
Achemical reaction is done in the setup shown, resulting in a change of mass. what will happen if the same reaction is done in a sealed container that is placed on the electronic balance?
Answers: 1
You know the right answer?
Find the new concentration of a solution if 25.0 ml of water is added to 125.0 ml of 0.150 m nacl so...
Questions


Mathematics, 21.03.2020 02:56

History, 21.03.2020 02:56



Mathematics, 21.03.2020 02:56

Biology, 21.03.2020 02:56


Computers and Technology, 21.03.2020 02:56

Mathematics, 21.03.2020 02:56

Mathematics, 21.03.2020 02:56


Social Studies, 21.03.2020 02:56

Chemistry, 21.03.2020 02:56


Mathematics, 21.03.2020 02:56

Engineering, 21.03.2020 02:56

Mathematics, 21.03.2020 02:56

Mathematics, 21.03.2020 02:56

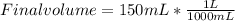
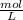 . It can be expressed as :
. It can be expressed as :